Simulation Worksheet - Atomic Spectra
Part A - General Questions
Please type your answers in the yellow shaded spaces.
1. Explain how we can identify an element based on its spectrum.
2. If we have two different elements, and each has an electron drop from the first excited state to the ground state, they will both have emission lines. But these lines may be very different between the two elements. Explain how this is possible.
Part B - Simulation Exercise
This is a simulation of a gas discharge tube in which electrons are used to excite atoms which then give off light. This light can be recorded by a spectrometer. Begin the simulation using the "One Atom" tab (top left corner). In the "Options" panel (lower right hand side of the screen), check the "Spectrometer" and "Squiggle" boxes. Notice the energy level diagram on the right of the screen, as well as the spectrum at the bottom of the screen (the spectrum is blank right now because we have not yet excited the atom).
One Atom
1. Fire a single electron towards the hydrogen atom by clicking the "Fire electron" button in the middle of the screen. Describe what happens in a step by step fashion. (Note: It may be helpful to utilize the "Run in slow motion" option found in the "Option" panel in the lower right hand corner)
2. After you have fired the electron, notice that a red arrow labeled "Energy at collision" has appeared in the energy level diagram. What does this mean? (i.e. this is the energy of "what" at the moment of collision)
3. How can you adjust where the "Energy at collision" arrow appears in the energy level diagram? Is there more than one way? Explain.
4. How is light "produced" by the atom in this simulation?
5. When the hydrogen atom is moved all the way to the left in the discharge tube, it is not possible to cause the emission of light. Why is this? (Hint: see your answer to question 3)
6. Is it possible for a single electron to collide with the atom and produce more than one photon of light? Explain. (Note: You might want to utilize the slow motion option)
Multiple Atoms
Although we could generate a complete spectrum using just one atom, the process is much faster using many atoms. Click on the "Multiple Atoms" tab in the top left corner. Make sure that the Spectrometer and Squiggle options are checked. You will notice that lots of electrons are exciting the atoms in the tube. You will also notice that spectral lines are appearing in the spectrometer at the bottom of the screen. The photons that are producing these lines can be seen as squiggles in the energy level diagram.
7. By observing the colors of the "squiggles" produced in the energy level diagram, state which color of photon has the most energy and explain why.
You can change the atom in the tube using a pull-down menu control labeled "Atom Type". For each of the gases listed in the pull-down menu (Hydrogen, Mercury, Sodium and Neon), record the colors of light in the visible spectrum emitted by each element in the table given below (simply type the capital letter "I", in bold, in the position of each spectral line in the table, and change the color of the "I" to the color of the line. Hydrogen has been done as an example in the table below). Note that the numbers along the bottom of the spectrometer have units of nm (nanometers), which are units of wavelength.
Table - Emission Spectra
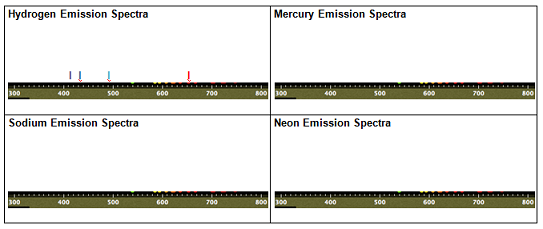
8. What is the relationship between wavelength and energy, based upon your observations? Explain using the spectra you obtained in the table.
Click on the "View Picture of Actual Discharge Lamps" button at the bottom of the screen. You should see a picture of gas discharge tubes containing the four elements we examined in this exercise.
9. Note the colors of the gasses in the picture. Could you predict what color a gas would be based on its emission spectrum? How? (Hint: What is the source of light emitted by the gas tubes?)