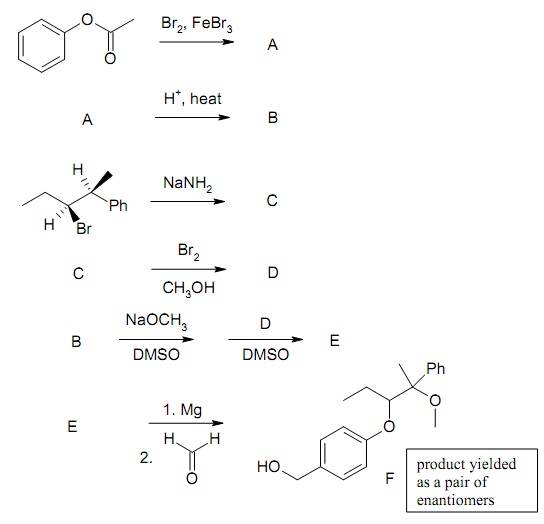
The given sequence of reactions was used to synthesis compound F. Compound B, which is generated in step 2, is used in the fifth step. In step 5, B is de-protonated by NaOCH3 in an acid-base reaction and reacted with D in a substitution reaction (a small amount of alkene is as well formed in this reaction which should be ignored). In the first step, only one isomer, A, is formed. Compound F yielded as a pair of enantiomers. For each of the six reactions, draw the main product (A-F) of the reaction. Exhibit all stereoisomers of A-F which are formed. When the starting material consists of some stereoisomers, clearly point out the stereoisomer of the product formed from each of the stereoisomers of the starting material.
Draw the accepted method for each reaction and describe how each stereoisomer is formed in the reaction. For the reaction, that yields product B, predict the peaks in the IR and NMR spectra of the starting material and the product B. As well comment on the differences in these spectra that point out a successful reaction yielding the product.