problem 1: The living polymerization of norbornene, catalyzed by A, can be halted by the addition of benzaldehyde. The resulting polymer is terminated at one end by -CH=CHPh and at the other by -CH=CHtBu. The byproduct is B.
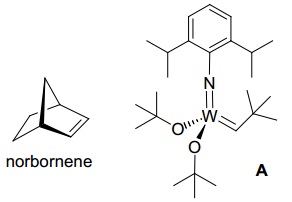
a) What type of catalyst is A?
b) What is a “living polymer”?
c) What is the driving force for the polymerization?
d) Draw the repeating unit of the polymer.
e) describe the chain termination step.
f) Draw the structure of B.
problem 2: Give a brief account of the chemistry:
a) Pauson-Khand reaction
b) Takasago stereoselective isomerization
c) Sonogashira reaction
d) Monsanto and Cativa processes
problem 3: Give an account of the uses, strengths and weaknesses of the different characterization methods generally used in organometallic chemistry.
problem 4: The transformation below is called hydroaminomethylation, first reported by Reppe over 60 years ago. There are four stages to this process, all of which occur in the same reaction vessel. Three of these are catalyzed by the same Rh catalyst, [Rh(I)L2]+ (L2 is a chelating bisphosphine). First, internal alkenes are isomerized to terminal alkenes. Next, the terminal alkene undergoes hydroformylation with a preference for formation of linear instead of branched aldehyde. Third, the aldehyde reacts with a primary amine (without catalysis) to give the imine, which undergoes Rh-catalyzed hydrogenation to the amine in the last step.
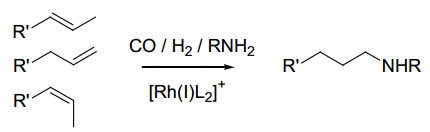
Propose mechanisms for:
a) The isomerization of internal to terminal alkenes
b) Hydroformylation
c) Hydrogenation of imine to amine.
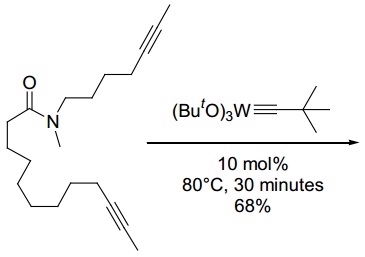
problem 5: Draw both products of the ring-closing alkyne metathesis reaction below, and show all steps of the mechanism. What is the driving force for the reaction? What are likely byproducts?
problem 6: Elaborate upon any THREE of the following subjects: (a) the Stille reaction, (b) Schrock alkylidenes and alkylidynes, (c) chiral phosphine ligands, (d) 1973 Nobel Prize in Chemistry, (e) dihydrogen complexes and agostic complexes, (f) self-healing polymers, (g) organometallic compounds in medicine and (h) organolanthanide chemistry.