Questions 1-4 relate to solution-state NMR spectroscopy.
Q1. Ortho-dichlorobenzene is an important intermediate in the synthesis of agrochemicals, dyes and pesticides. The 1H NMR spectrum of a purified sample of this compound measured on a 300 MHz NMR instrument is shown below:
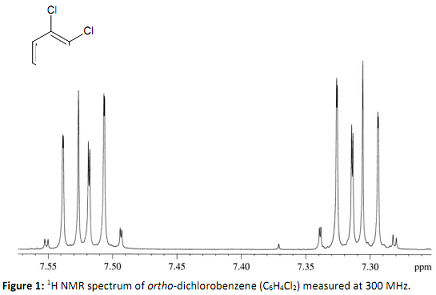
Briefly explain in a few sentences (and with a sketch of the molecule if necessary) why this spectrum is second order, i.e. the multiplet structures cannot be interpreted by the rules for first order NMR spectra (2nI+1 rule and Pascal's triangle rule for spin-spin splittings).
Would you expect this spectrum to simplify to a first order spectrum if it was rerun on a higher field instrument (e.g. 800 MHz)? Give a short (one or two sentences) explanation of your answer.
Q2. The spectra in Figure 2 are proton-decoupled carbon-13 NMR spectra of the three isomers of dichlorobenzene:
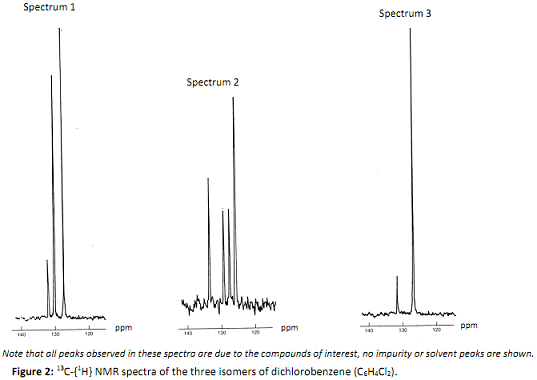
Identify which of the three possible isomers A, B or C has produced each spectrum.
Give a short justification for your answers. Hint: arguments based on chemical shift are not required to answer this question and should be avoided.
Q3. A compound with the formula C5H10O gives the following 1H NMR spectrum (Figure) and 13C NMR data (Table 1):
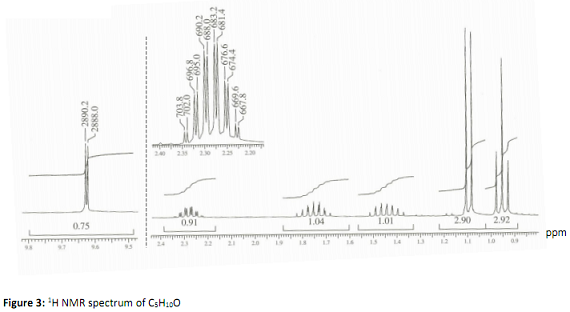
Notes: The (relative) size of the integrals are written directly below the proton peaks (i.e. you do not need to measure the integral heights with a ruler here!). Round these numbers to the nearest whole number.
The proton resonances at ca. 9.63 ppm and ca. 2.28 ppm have been peak picked. The peak picking shows the line positions in units of Hz, these data can be used to establish coupling constants.
Table 1: Tabulated 13C-{1H} and DEPT 135 and DEPT 90 NMR data for C5H10O
|
Observed Carbon-13 Chemical Shifts
|
DEPT 135
|
DEPT 90
|
|
11.35 ppm
|
Positive peak
|
No peak at this position
|
|
12.88 ppm
|
Positive peak
|
No peak at this position
|
|
23.55 ppm
|
Positive peak
|
No peak at this position
|
|
47.78 ppm
|
Positive peak
|
Positive peak
|
|
205.28 ppm
|
Positive peak
|
Positive peak
|
Notes: The tabulated data indicate the 13C chemical shifts observed in the 13C-{1H} NMR spectrum for C5H10O and the appearance of the DEPT 135 and DEPT 90 spectra at those peak positions. No other peaks were observed in these spectra (with the exception of the solvent peak in the 13C-{1H} spectrum).
What structural information can you deduce from the 1H, 13C and DEPT information given? Suggest and justify a structure for C5H10O which you believe best fits the NMR data.
Label the 1H resonances in Figure 3 and assign these labels to your structure.
Why are the protons with chemical shifts between 1.3 and 1.9 ppm inequivalent (comment with respect to the structure you have drawn!)?
Hint for question 3: Data for the most downfield resonance (1H and 13C) is diagnostic of a particular functional group - what is it?
Q4. Substituted indoles are important substructures in active pharmaceutical ingredients, with the substitution pattern being highly important for the efficacy of the material. A synthesized indole has one of the four possible isomers shown in Figure 4 below:
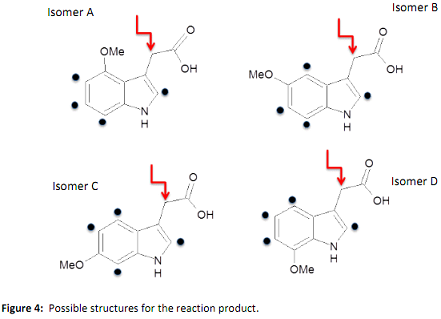
In order to determine the correct isomer, an NOE experiment was run irradiating the methylene protons (as indicated by the red arrow in Figure 4). Selected NMR data for the product is shown in Figure 5:
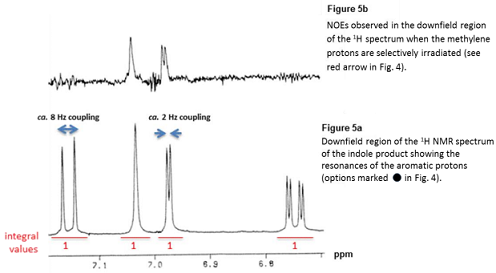
Notes: i) The OH and NH protons are exchangeable protons and are not observed; coupling to these protons is not observable; ii) The up-field region of the 1H NMR spectrum contains a singlet for the methylene protons (integral value 2) and a singlet for the methoxy protons (integral value 3), as expected.
Briefly (one sentence) describe the information obtainable from an NOE experiment.
Which is the correct isomer for the substituted indole?
Justify your answer. As part of your answer you should label the resonances in Figure 5a, transfer these labels to your structure and state how you have deduced these assignments.
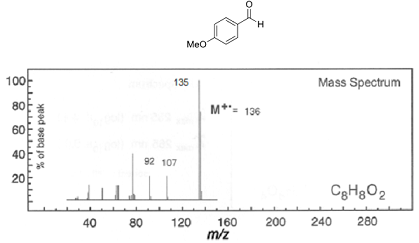
Q5. 4-Methoxybenzaldehyde can be used as a redox additive to assist in the degradation of pollutants by the fungus Pleurotus eryngii. Assign the labelled ions present in the following electron impact mass spectrum of 4-methoxybenzaldehyde by interpretation of the fragmentation pathway. Marks will be given for sensible structures and fragmentation mechanisms (showing curly arrows).
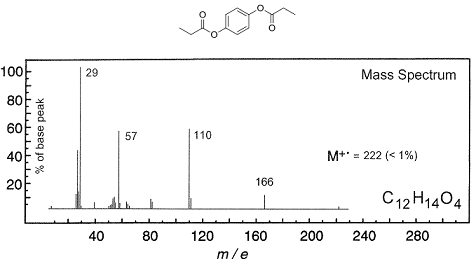
Q6. 1, 4-Phenylene dipropionate is the di-butanoate ester of hydroquinone, a component of sustainable Li-ion batteries. Assign the labelled ions present in the following electron impact mass spectrum of 1,4-phenylene dipropionate by interpretation of the fragmentation pathway. Marks will be given for sensible structures and fragmentation mechanisms (showing curly arrows).
Q7. Electrospray ionisation and collision-induced fragmentation of a pentapeptide (peptide containing 5 amino acid residues) yielded a mass spectrum containing the following series of y- and b-type ions.
|
Molecular Species of peptide [M+H] = 577.28
|
|
y-type ions
|
b-type ions
|
|
y1
|
175.12
|
b5
|
559.27
|
|
y2
|
306.16
|
b4
|
403.17
|
|
y3
|
421.19
|
b3
|
272.13
|
|
y4
|
478.21
|
b2
|
157.10
|
|
y5
|
577.28
|
b1
|
100.08
|
Given the reference list of condensed masses for amino acid residues below identify the sequence of the pentapeptide. Remember to provide the amino acid sequence of the peptide from N to C terminus, as by convention.
|
Reference List of condensed Amino Acid (AA) Masses
|
|
AA
|
mass/amu
|
AA
|
mass/amu
|
|
Ala
|
71.04
|
Leu
|
113.08
|
|
Arg
|
153.10
|
Lys
|
128.10
|
|
Asn
|
114.04
|
Met
|
131.04
|
|
Asp
|
115.03
|
Phe
|
147.07
|
|
Cys
|
103.01
|
Pro
|
97.05
|
|
Glu
|
129.04
|
Ser
|
87.03
|
|
Gln
|
128.06
|
Thr
|
101.05
|
|
Gly
|
57.02
|
Trp
|
186.08
|
|
His
|
137.06
|
Tyr
|
163.06
|
|
Ile
|
113.08
|
Val
|
99.07
|