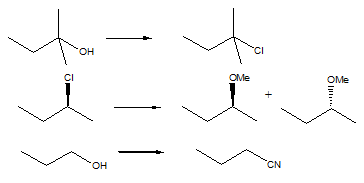
Questions -
Q1. Predict the major product(s) of each of the following reactions. Show stereochemistry where important:
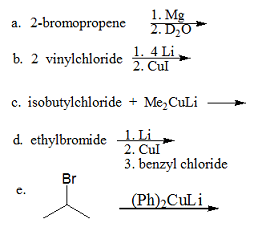
Q2. Complete the following reactions by drawing the major products A-C.
Q3. How would you carry out the following transformations? Show all reagents and intermediates along the way.
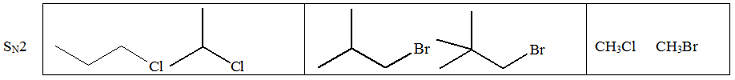
Q4. A. Circle the substrate in each box that would react faster in the indicated substitution reaction.
B. Circle the stronger nucleophile in each pair below:
a. NH3 or PH3
b. CH3OH or CH3OΘ
c. CH3COOΘ or CH3OΘ
d. ΘNH2 or ΘOH
C. Select the reaction that proceeds faster? Explain your choice!
(CH3)3CCl + H2O → (CH3)3COH
(CH3)2C=CHCl + H2O → (CH3)2C=CHOH
D. Rank these substances according to the rate of precipitation of NaCl when treated with a solution of NaI in acetone (fastest to slowest).
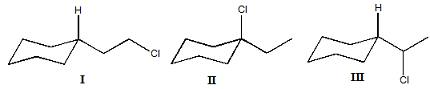
E. Rank these substances according to the rate of solvolysis in aqueous ethanol (fastest to slowest).
I. 3-bromo-1-butene
II. isopropyl bromide
III. methyl bromide
F. Which of the following solvents is aprotic?
I. CH3CH2OH
II. CH3CH2NHCH3
III. CH3CH2OCH2CH3
IV CH3CO2H
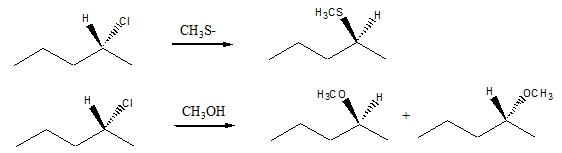
Q5. Consider the two reactions below for the optically active alkyl halide, (R)-2-chloropentane. The first reaction occurs rapidly at room temperature and gives an optically active product. The second reaction, starting with the same alkyl halide, occurs slowly at room temperature and gives a non-optically active product (racemic mixture). Draw a mechanism (include all H's and lone pairs) for each reaction and explain how the mechanism leads to the observed results.
Q6. Predict the product(s) of each of the following reactions include stereochemistry where important and indicate the mechanism (SN1, SN2).
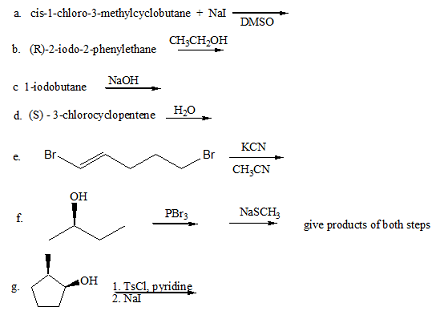
Q7. Provide the reagents necessary to achieve the following