Question: Another experiment was performed to validate the fact that the vessel in Example was well mixed. In this experiment, the vessel was well insulated and brought to steady state. Then a step change was introduced to the inlet temperature. The following data represents the operating conditions, and the dynamic data is given .
Data: V = 2.7 m3, F = 0.71 m3/min., T0init = 103.5°C, T0 = 68°C.
(a) Formulate the energy balance for this system, and solve for the expected dynamic response of the tank temperature.
(b) Compare your prediction with the data.
(c) Given the two experimental results in Figure and this question for the same equipment, discuss your conclusions on the assumption that the system is well mixed.
(d) Is there additional information that would help you in (c)?
Example: Validation. The mixing tank was built, the experiment was performed, and samples of the outlet material were analyzed. The data points are plotted in Figure 3.4 along with the model prediction. By visual evaluation and considering the accuracy of each data point, one would accept the model as "valid" (or, more accurately, not invalid) for most engineering applications. The modelling procedure presented in this section is designed to ensure that the most common issues are addressed in a logical order. While the procedure is important, the decisions made by the engineer have more impact on the quality of the result than the procedure has. Since no one is prescient, the effects of early as sumptions and formulations may not be appropriate for the goals. Thus, a thorough analysis of the results should be performed so that the sensitivity of the conclusions to model assumptions and data is clearly understood. If the conclusion is unduly sensitive to assumptions or data, an iteration would be indicated, employing a more.
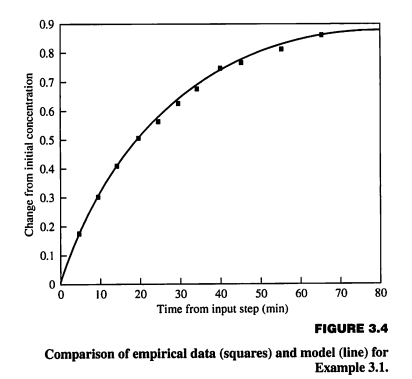
rigorous model or more accurate data. Thus, the procedure contains the essential opportunity for evaluation and improvement.