Question 1: The reaction X + Y → products was studied using the method of initial rates. The initial rate of consumption of X was measured in three different experiments. Data are provided below.
|
Expt
|
[X] o
(in mol/L)
|
[Y] o
(in mol/L)
|
Initial Rate
(in mol L -1 s - 1 )
|
|
1
|
0.500
|
0.400
|
0.8600
|
|
2
|
1.50
|
0.400
|
0.8600
|
|
3
|
0.500
|
1.20
|
2.580
|
What is the value of the rate constant, k? (Use concentrations in mol/L and time in seconds. Enter your answer accurate to three significant figures.)
Question 2: The reaction
2I-(aq) + H3AsO4(aq) + 2H+(aq) → I2(aq) + H3AsO3(aq) + H2O(l)
proceeds via the mechanism below. According to the given mechanism, what is the correct expression for the rate of consumption of H3AsO4?
Step 1 (reversible): H3AsO4 + H+ ? H4AsO4+ fast in both directions, with rate constants k1 and k-1
Step 2 (irreversible): H4AsO4+ + I- ? H3AsO3 + HOI slow, with rate constant k2
Step 3 (irreversible): HOI + I- + H+ ? I2 + H2O fast, with rate constant k3
(a) Rate=k [I-]2 [H3AsO4] [H+]2/[H3AsO3]
(b) Rate = k [I-]2 [H3AsO4] [H+]
(c) Rate = k [I-] [H3AsO4] [H+]2
(d) Rate = k [I-]2 [H3AsO4] [H+]2
(e) Rate = k [I-]2 [H3AsO4]
(f) Rate = k [I-] [H3AsO4] / [H+]
(g) Rate = k [I-] [H+]2
(h) Rate=k [H3AsO3] / ([I-]2 [H3AsO4] [H+]2)
(i) Rate = k [I-] [H3AsO4]
(j) Rate = k [H3AsO4] [H+]
(k) Rate = k [I-]2 [H+]
(l) Rate = k [I-] [H3AsO4] [H+]
Question 3: The reaction X + Y → products was studied using the method of initial rates. The initial rate of consumption of X was measured in three different experiments. Data are provided below.
|
Expt
|
[X]o
(in mol/L)
|
[Y]o
(in mol/L)
|
Initial Rate
(in mol L-1 s-1)
|
|
1
|
0.700
|
0.400
|
6.608
|
|
2
|
2.10
|
0.400
|
19.82
|
|
3
|
0.700
|
1.20
|
59.47
|
What is the order of the reaction with respect to X? ____________ (Enter an integer.)
What is the order of the reaction with respect to Y? ____________ Enter an integer.)
Specify the units of k by giving the appropriate powers of mol, L and s:
The power of mol is ____________ (Enter an integer.)
The power of L is ____________ (Enter an integer.)
The power of s is ____________ (Enter an integer.)
Question 4: Consider the following reaction:
3 A(aq) + 4 B(aq) → 4 C(aq) + 4 D(aq)
What is the rate of production of C, in mol L-1 s-1, if the rate of consumption of A is 0.790 mol L-1 s-1?
Enter your answer accurate to three significant figures. Do not include units. If necessary, use exponential notation (e.g. 1.23E-3 for 1.23x10-3).
Question 5: The decomposition reaction, X → products, is zeroth-order with rate constant k=0.0811 mol L-1 s-1. How long will it take for the decomposition to be 95% complete, if the initial concentration of X is 1.25 mol/L?
Give your answer in seconds, accurate to three significant figures. Do not include units as part of your answer.
Question 6: The decomposition reaction X → products was studied as a function of time. Data are given in the table below.
|
time (in s)
|
[X] (in mol/L)
|
|
0.0
|
1.650
|
|
3.20E2
|
0.8259
|
|
6.40E2
|
0.5508
|
|
9.60E2
|
0.4131
|
|
1.28E3
|
0.3306
|
What is the value of the rate constant? (Give your answer accurate to three significant figures. Do not give units as part of your answer, but use concentrations in mol/L and time in seconds.)
Question 7: Consider the data below for the reaction X → products. (Hint: You will have to determine the order of the reaction before you can answer parts (b)-(d) of this question.)
|
Time (in seconds)
|
[X] (in mol/L)
|
|
0
|
1.5500
|
|
3.60E2
|
0.7755
|
|
7.20E2
|
0.5171
|
|
1.08E3
|
0.3878
|
|
1.44E3
|
0.3103
|
For parts (a), (c), and (d): Enter your answer accurate to three significant figures. Do not include units in your answer, but use concentrations in mol/L and time in seconds.
(a) What is the average rate of consumption of X between 3.60E2 s and 1.08E3 s?
(b) What is the order of the reaction? (Enter an integer, or a fraction, e.g. 1/2, 3/2, etc.)
(c) What is the value of the rate constant, k?
(d) What is the instantaneous rate of consumption of X at 1.08E3 s?
Question 8: The activation energy, Ea, for a second-order reaction, X →products, is most readily obtained from the slope of a plot of
(a) [X] versus time
(b) k versus 1/T, where T is temperature in K
(c) ln k versus time
(d) k versus T, where T is temperature in K
(e) ln k versus T, where T is temperature in K
(f) [X]2 versus time
(g) 1/[X] versus time
(h) Rate versus time
(i) ln[X] versus time
(j) ln k versus 1/T, where T is temperature in K
Question 9: Fill in the blanks:
The elementary reaction A(aq) + B(aq) → C(aq) is exothermic in the forward direction. What happens to k1, k-1 and Kc when the temperature increases? (In each case, type one of the following: increases, decreases , or remains the same .
(a) k1: ____________ .
(b) k-1: ____________ .
(c) Kc: ____________ .
Question 10: For the elementary process below, the activation energy is 41 kJ/mol and the enthalpy change is 22 kJ/mol. What is Ea for the reverse process, in kJ/mol?
A + B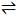 AB
AB
Question 11: A reaction has a first order rate constant of 4.09x10-5 s-1 at 25oC and 0.00141 s-1 at 77oC. What is the value of the rate constant at 55oC?
Enter your answer in units of s-1, accurate to three significant figures. Do not include units as part of your answer. (Enter 1.23x10-3 as 1.23e-3.)
Question 12: A catalyst decreases the activation energy of a given reaction by exactly 13 kJ/mol. By what factor does the reaction rate increase at 25oC when the catalyst is used? Assume that the Arrhenius pre-exponential factor, A, is the same for the catalyzed and uncatalyzed reactions.
Enter your answer accurate to three significant figures. Do not include units as part of your answer. (Enter 1.23x10-3 as 1.23e-3.)
Question 13: Consider the reaction profile below.
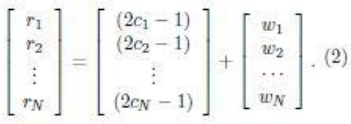
Which step has the smallest rate constant?
(a) A --> B
(b) B --> C
(c) B --> A
(d) C --> B