Question 1
1. Hyaluronidases (HYAL) are family of enzymes that degrade hyaluronic acid (HA). HA is an important glycosaminoglycan in connective and other tissues in the body. HYAL I and II have been implicated as a potential drug target in allergy-related asthma. Your PI decides that he wants to run a functional assay with HYAL cleaving HA.
(A) Determine which protein expression system(s) would be appropriate for producing the protein.
(B) Determine which techniques are available purify the protein. Select one and justify why you choose this technique.
(C) Determine which techniques are available to determine protein purity. Select which techniques you will use and justify your selection.
Question 2
1. Interleukin-2 (IL2) is a cytokine that regulates white blood cells. IL2 and IL2R are commonly used as a model drug target for technique development. Describe what techniques could elucidate the primary, secondary, and tertiary structure of this protein and its receptors. In addition, describe what techniques could be used to characterize the binding of IL2 with IL2R. Hint: often, multiple techniques are used to validate results.
Question 3
1. You work in a lab that studies spider silk, and you are in charge of silking the spiders for their protein. Your PI asks you to characterize the silk you are harvesting. Describe ways you can characterize it, and what equipment you need to do so.
Question 4
Glycoprotein D of Herpes simplex virus (HSV) is a membrane fusion protein that has become a target for therapeutic antibody and vaccine development.
E317 is an antibody that was developed to neutralize HSV. Recently, a crystal structure of the interaction between E317 and gD was determined.
Describe the functional groups on the amino acids for the noncovalent bonds between the molecules.
Using these same functional groups describe the bonds between the functional groups.
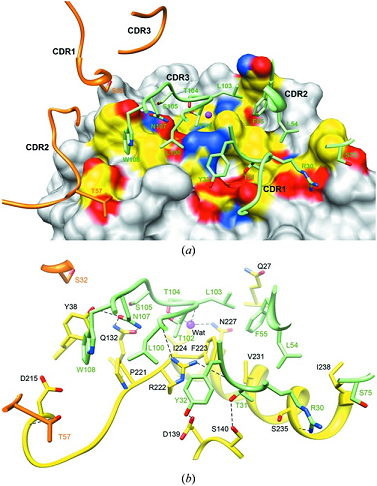
Question 5
1. IL2-Receptor (IL-2R) is a receptor of IL-2 that is expressed in lymphocytes. Below is the crystal structure of IL-2 and IL-2R bound.
(A) Describe the different noncovalent bonds that exist in the graphic. Be sure to describe in terms of both functional groups and amino acids.
(B) Describe how you would characterize the binding of the two proteins.(Remember that multiple techniques are commonly used as validation).
(C) List at least five buffers that would be necessary to test when characterizing the binding in part B and why.
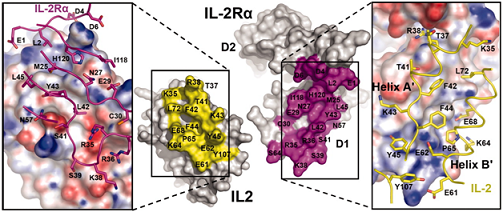
Question 6
Glycoprotein D (gD) of Herpes Simplex Virus bind nectin-1 (a ubiquitous cell membrane receptor) at sites Tyr38, Asp215, and Arg222.
These residues and Phe223 have also been identified as major viral neutralization sites.
Identify five potential mutations that could replace these residues resulting in a change in either structure or function.
Offer an explanation through a lens of the functional groups on the amino acids for each answer.
Question 7
Spider silk is being explored for applications ranging from bullet proof vests to ligament replacement due to its strength and elasticity.
Silk is composed of two proteins, MaSP1 and MaSP2. Silk is not very soluble.
Given the following table describing the amino acid composition of some silk proteins, describe why it is insoluble and identify buffers that could be used to solubilize silk.
|
Gly
|
Ala
|
Pro
|
Glx
|
Ser
|
Tyr
|
Leu
|
Val
|
Thr
|
Asx
|
Arg
|
Phe
|
|
Araneus gemmoides
|
42.8
|
19.4
|
11.1
|
8.2
|
7.1
|
5.2
|
1.1
|
1.1
|
1.0
|
0.9
|
0.6
|
0.5
|
|
Argiope argentata
|
44.6
|
19.3
|
10.2
|
9.2
|
6.8
|
5.4
|
1.0
|
0.5
|
0.5
|
0.4
|
1.1
|
0.8
|
|
Argiope aurantia
|
46.4
|
17.9
|
9.5
|
9.4
|
5.9
|
4.8
|
1.5
|
0.7
|
0.5
|
0.5
|
1.2
|
0.9
|
|
Latrodectus hesperus
|
45.7
|
31.1
|
1.5
|
8.7
|
1.1
|
4.5
|
0.7
|
0.6
|
0.7
|
0.7
|
1.5
|
0.4
|
|
Nephila clavipes
|
47.1
|
26.5
|
1.2
|
8.8
|
3.0
|
3.6
|
4.2
|
1.1
|
0.6
|
0.6
|
1.2
|
0.3
|
Asx = aspartate and aspartic acid, Glx = glutamine and glutamate. The variability in composition within each spider silk fiber sample was observed to be large (up to 50%) and the values presented are the average of five analysis runs on five different samples of each spider silk.