Q1. Tricostatin A (TSA) is a protein deacetylase inhibitor. You know that HDACs remove acetyl groups from lysines. You are interested in identifying how TSA works. You do a number of experiments. You find the level of a protein, called survivin is increased 10-fold in cells when you treat with TSA. You assume this is at the level of transcription. You measure the mRNA for survivin and find it is unchanged. Can you think why TSA increased survivin protein?
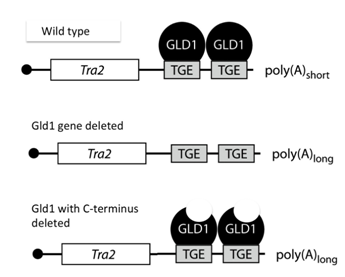
Q2. mRNAs have different half-lives in the cells. This is regulated, in part by sequences in the 3' UTR, the elements are labeled as TGE. You place the mRNA shown in wild type cells, a cell containing a deletion of GLD1 and another with a version of GLD1 where the last 100 amino acids on the c-terminus of the proteins is removed. You then analyzed the polyA length and abundance of the mRNA.
a. What is the function of GLD1 in the cells?
b. What factors do you think GLD1 would bind to and bring to the mRNA?
c. Would deleting GLD1 increase translation of tra2? Defend your answer
d. Finally, what can you conclude about the C-terminus of GLD1
e. tough question: Suppose GLD1 is an essential gene and cells could not live without it. Is there a way to test if it regulates the mRNA even if you have to do experiments in cells containing the wild type gene?
3. Us e the figure below to answer questions A-D. You are interested in understanding the gene regulation of Lkp1, a protein that is normally produced in liver and kidney cells in mice. Interestingly, you find that the LKP1 gene is not expressed in heart cells. You isolate the DNA upstream of the LKP1 gene, place it upstream of the gene for green fluorescent protein (GFP), and insert this entire piece of recombinant DNA into mice. You find GFP expressed in liver and kidney cells but not in heart cells, an expression pattern similar to the normal expression of the LKP1 gene. Further experiments demonstrate that there are three regions in the promoter, labeled A, B, and C in the figure, that contribute to this expression pattern. Assume that a single and unique transcription factor binds each site such that protein X binds site A, protein Y binds site B, and protein Z binds site C. You want to determine which region is responsible for tissue-specific expression, and create mutations in the promoter to determine the function of each of these regions. In the figure below, if the site is missing, it is mutated such that it cannot bind its corresponding transcription factor.
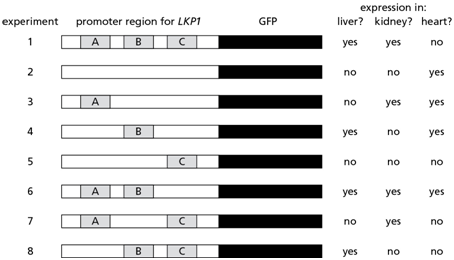
A. Which of the following proteins is likely to act as a gene repressor?
(a) factor X
(b) factor Y
(c) factor Z
(d) none of the above
B. Which of the following proteins are likely to act as gene activators?
(a) factors X and Y
(b) factors X and Z
(c) factors Y and Z
(d) factor X only
C. Experiment 1 in the figure is the positive control, demonstrating that the region of DNA upstream of the gene for GFP results in a pattern of expression that we normally find for the LKP1 gene. Experiment 2 shows what happens when the sites for binding factors X, Y, and Z are removed. Which experiment above demonstrates that factor X alone is sufficient for expression of LPK1 in the kidney?
(a) experiment 3
(b) experiment 5
(c) experiment 6
(d) experiment 7
D. In what tissue is factor Z normally present and bound to the DNA?
(a) kidney
(b) liver
(c) heart
(d) none of the above
4. Below is a chart of pI (Isoelectric Point) and molecular weight of four proteins:
|
Protein
|
Molecular Weight (kDa)
|
pI
|
|
Histone H3
|
15
|
11.1
|
|
Myoglobin
|
17
|
7.2
|
|
BSA
|
68
|
4.7
|
|
Myosin
|
200
|
5.2
|
A. Cation exchange chromatography uses negative charges to retain positively charged proteins. You run a mixture of the proteins above on a cation exchange column at pH = 7.2. What proteins will be retained?
B. What sequence do you expect the mixture of proteins to elute (first to last) when you run the mixture on a size exclusion column?
C. If you run the mixture on an SDS page, which protein will migrate most rapidly in the gel? Which one most slowly?
D. Native PAGE works similarly to SDS PAGE except that proteins are kept under non-denaturing conditions so that the proteins' secondary structure and native charges are maintained. If you run the mixture above on a native PAGE with buffers at pH = 7.2, which direction will these proteins migrate towards- the positive pole (anode) or the negative pole (cathode)- when an electrical current is applied?