Q1. Draw the Lewis structures for CO2 and CO, and predict the number of σ and π bonds for each molecule.
(a) CO2
(b) CO
Q2. What is the hybridization of the central atom in each of the following?
(a) BeH2
(b) SF6
(c) PO43-
(d) PCl5
Q3. Two important industrial chemicals, ethene, C2H4, and propene, C3H6, are produced by the steam (or thermal) cracking process:
2C3H8(g) ? C2H4(g) + C3H6(g) + CH4(g) + H2(g)
For each of the four carbon compounds, do the following:
(a) Draw a Lewis structure.
(b) Predict the geometry about the carbon atom.
(c) Determine the hybridization of each type of carbon atom.
Q4. For the carbonate ion, CO32-, draw all of the resonance structures. Identify which orbitals overlap to create each bond.
Q5. For each of the following structures, determine the hybridization requested and whether the electrons will be delocalized:
(a) Hybridization of each carbon
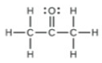
(b) Hybridization of sulfur
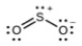
(c) All atoms
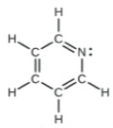
Q6. Calculate the bond order for an ion with this configuration:
(σ2s)2(σ*2s)2(σ2px)2(π2py,π2pz)4(π*2py,π*2pz)3
Q7. Predict the valence electron molecular orbital configurations for the following, and state whether they will be stable or unstable ions.
(a) Na22+
(b) Mg22+
(c) Al22+
(d) Si22+
(e) P22+
(f) S22+
(g) F22+
(h) Ar22+
Q8. For the first ionization energy for an N2 molecule, what molecular orbital is the electron removed from?