problem 1: F3CI has a δ– CF3 group and a δ+ I. Since of this, to make trifluoromethyl complexes of transition metals, F3CC(O)Cl is frequently used. How does this approach work?
problem 2: Metal alkoxides, such as metal alkyls, can as well β-eliminate. With this in mind:
a) Describe why –OtBu is a common ligand in metal alkoxide chemistry.
b) What are the products of decomposition of primary and secondary alkoxide ligands?
c) How can alcohols, in the presence of a base, be employed as reducing agents for metal complexes?
problem 3: Mo(CO)6 undergoes substitution reactions with phosphine ligands, however the reaction never proceeds further than the Mo(CO)3(PR3)3 stage. If the phosphines are much bulky, the phosphines are arranged mer, however otherwise are always fac. Describe these two observations.
problem 4: CpRe(NO)(CO)Me reacts with two equivalents of PMe3 to give a product in which six ligands are bound to Re. The reaction has large negative ΔS‡. Draw the product and recommend a plausible mechanism.
problem 5: In the substitution of V(CO)6, the rate of reaction modifies with respect to phosphine nucleophile according to the order PMe3 > PBu3 > P(OMe)3 > PPh3. What does this recommend regarding the mechanism?
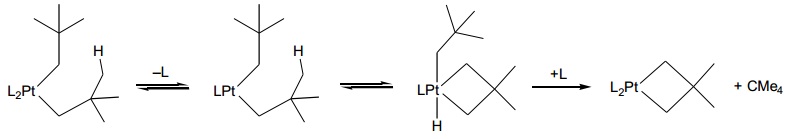
problem 6: In the given reaction scheme, name the reaction(s) taking place at each step and work out the oxidation state and electron count of all metal complexes.