Find the ka (acid equilibrium constant) for the acid.
Formic acid is the simplest carboxylic acid. It is an important intermediate in chemical synthesis and occurs naturally, must notably in the vendom of the bee and ant stings. In fact, its name comes from the latin word for ant, formica, referring to its early isolation by the distillation of any bodies.
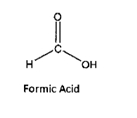
If the pH of a 0.30 molar solution of formic acid is 2.02 what is the ka (acid equilibrium constant) for the acid?