Compute the structural analysis of the protein.
1. The chirality of an amino acid results from the fact that its carbon:
i. has no net charge.
ii. is a carboxylic acid.
iii. is bonded to four different chemical groups.
iv. is in the l absolute configuration in naturally occurring proteins.
v. is symmetric.
2. Two amino acids of the standard 20 contain sulfur atoms. They are:
a. Cysteine and serine.
b. Cysteine and threonine.
c. Methionine and cysteine.
d. Methionine and serine.
e. Threonine and serine.
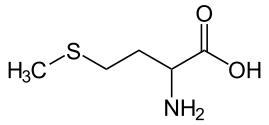
3. Which of the following statements about cystine is correct?
a. Cystine forms when the -CH2-SH R group is oxidized to form a -CH2-S-S-CH2- Disulfide Bridge between two cysteines.
b. Cystine is an ex of a nonstandard amino acid, derived by linking two standard amino acids.
c. Cystine is formed by the oxidation of the carboxylic acid group on cysteine.
d. Cystine is formed through a peptide linkage between two cysteines.
e. Two cystines are released when a -CH2-S-S-CH2- disulfide bridge is reduced to -CH2-SH.
4. Titration of valine by a strong acid, for ex HCl, reveals two pK's. The titration reaction occurring at pK2 (pK2 = 9.74)is:
a. -COO + H → -COOH
b. -COOH + -NH2 → -COO + -NH2+
c. -COO + -NH2+ → -COOH + -NH2
d. -NH2 + H → -NH3+
e. -NH + H → -NH2
5. For amino acids with neutral R groups, at any pH below the pI of the amino acid, the population of amino acids in solution will have:
a. a net negative charge.
b. a net positive charge.
c. no charged groups.
d. no net charge.
e. positive and negative charges in equal concentration.
6. At pH 8, the functional groups of histidine (pKa's are α-carboxylate=1.8, α-amino=9.3, imidazole=6.0)would be charged as follows:
a. -1 α-carboxylate, 0 α-amino, +1 imidazole = 0 net charge.
b. -1 α-carboxylate, +1 α-amino, +1 imidazole = +1 net charge.
c. -1 α-carboxylate, 0 α-amino, 0 imidazole = -1 net charge.
d. -1 α-carboxylate, +1 α-amino, 0 imidazole = 0 net charge.
e. α-carboxylate, +1 α-amino, +1 imidazole = +2 net charge.
7. What is the pI of aspartic acid? (use the pKa values of α-carboxylate=2.0, α-amino=10.0, β-carboxylate=4.0)
a. 7
b. 3
c. 1
d. 8
e. 4
8. In a protein experiment, the absorbance of a protein sample at 280 nm was 2.5. Using a molar extinction coefficient of 0.5/cmM, and a path length of 1 cm, find out the concentration of this protein in the sample.
a. 0.5 M
b. 3 M
c. 1.25 M
d. 5 M
e. 5 mM
9. Which of the following methods does NOT fractionate by size?
i. gel filtration chromatography
ii. isoelectric focusing
iii. SDS-PAGE
iv. gradient ultracentrifugation
v. all fractionate by size
10. Which of the following methods does NOT fractionate by charge?
a. action exchange chromatography
b. anion exchange chromatography
c. affinity chromatography
d. isoelectric focusing
e. only a & b