problem: Consider the process of figure with the stream temperatures and the heat duties given on table shown below:
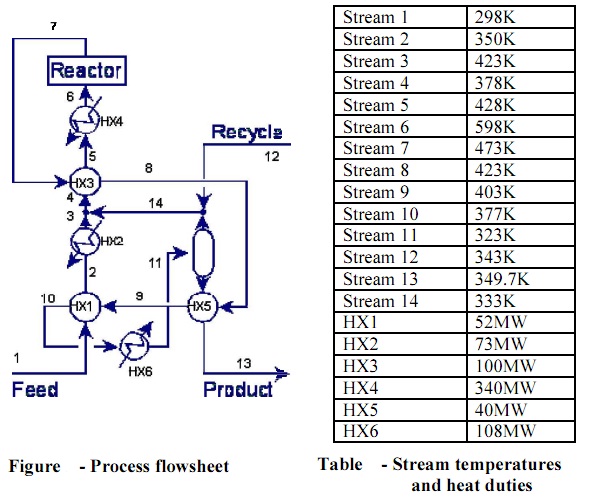
The flash drum operates at 323K.
a) Extract stream data from the process of Figure shown below. Prepare the stream table for energy integration, presenting the number and the types of the hot and the cold streams. For each stream, indicate supply temperatures, target temperatures, and heat capacities to use for energy integration.
b) Using a minimum approach temperature of ?Tmin = 20K, draw the composite curves for the process given and determine the minimum heating and cooling demands.
c) Develop a heat exchanger network to match the minimum energy consumption Process and Energy Integration – Spring 2013
problem: The problem table for a process is given below for ?Tmin = 20K.
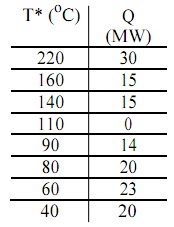
a) Draw the grand composite curve for this process.
b) A simple distillation column is to be integrated into the process. The relevant design information for the column is summarized below:
Condenser temperature (C) TBP = 93.2(P(bar))0.241
Reboiler temperature (C) TBP = 121.9(P(bar))0.267
Reboiler duty = 12MW
Condenser duty = 12MW
Column Design Pressure (P) = 2 bar
The change in condenser and reboiler duties with temperature is assumed to be negligible.
i) Determine the minimum heating and cooling requirements of the process if the column is integrated with the process. Illustrate your answer in the Grand Composite Curve.
ii) Suggest changes to the column specification that would reduce the minimum heating and cooling requirements of the process when the column is integrated with the process. Illustrate your answer in the Grand Composite Curve.