Chemistry Assignment
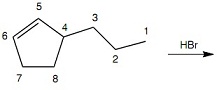
Question 1: What carbon atom will be bonded to a bromine atom in the major organic product(s) of Reaction 1? Reaction 1
Question 2: Which carbon atom bears the positive charge in the carbocation that arises from the rearrangement implied by the curved arrow notation shown in Figure 1?
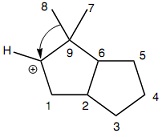
Figure 1
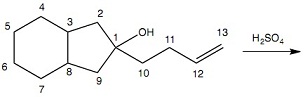
Question 3: What carbon atom will be bonded to an oxygen atom in the major organic product(s) of Reaction 2? Reaction 2
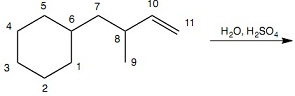
Question 4: What carbon atom will be bonded to a chlorine atom in the major organic product(s) of Reaction 3? Reaction 3
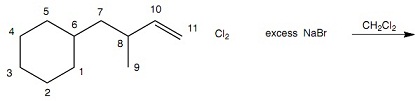
Question 5: How many organic products are formed in Reaction 4? Hint / The alkenes react sequentially, rather than at the same time. Each structural isomer counts as one organic product. Each stereoisomer counts as one organic product.
Reaction 4
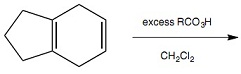
Question 6: Which one of the following statements about Reaction 5 is correct?
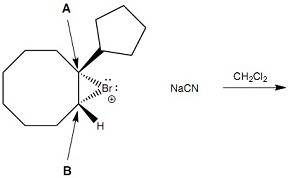
Reaction 5
- The major organic product arises from front face attack by cyanide ion at atom A
- The major organic product arises from front face attack by cyanide ion at atom B
- The major organic product arises from back face attack by cyanide ion at atom A
- The major organic product arises from back face attack by cyanide ion at atom B
Question 7:What carbon will be bonded to an oxygen atom in the major organic product(s) of Reaction 6? Reaction 6
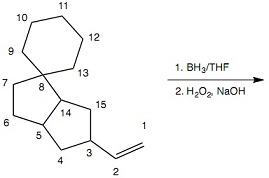
Question 8: Regarding Reaction 7 which of the labeled carbons become sp2 carbons of C=O groups of aldehydes? Select all that apply.
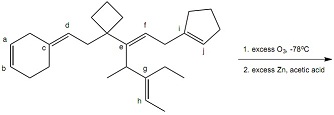
Reaction 7
Question 9: How many organic products are formed in Reaction 8? Hint / The alkenes react sequentially, rather than at the same time. Each structural isomer counts as one organic product. Each stereoisomer counts as one organic product.
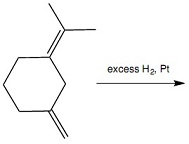
Reaction 8
Question 10: What carbon atoms will be bonded to an oxygen atom in the major organic product(s) of Reaction 9? Select all that apply.
Reaction 9