Use the following information to answer 1-2:
• Gasoline can be represented by the empirical formula CH2.
• There are 6.0 × 108 automobiles in the world.
• On average, 95% of the carbon in gasoline is converted to CO2.
• Average fuel efficiency is 11 L/100 km.
• Average distance travelled per car is 1.7 × 104 km/yr.
• The mean density of gasoline is 800 g/L.
• Annual CO2 emissions from fossil fuel combustion are 7.18 Pg C/yr.
1. For what fraction of annual CO2 emissions from fossil fuel burning are automobiles responsible?
2. A 1000-kg car travels 1.6 × 104 km/yr. What is the ratio of the mass of CO2 produced by fuel combustion to the mass of the car, if the car’s fuel economy is:
A. 5.0 L/100 km
B. 7.5 L/100 km
C. 10 L/100 km
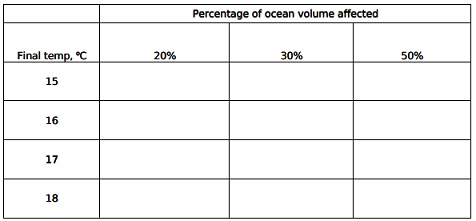
3. find out expected sea level rise for all possible combinations (shown in table below) of temperature increase and volume affected. Base your calculations on the following information and assumptions. Neatly lay out one ex calculation; do not show all calculations. Present your find outd values neatly in the table.
• Total ocean volume is exactly 1.36 × 109 km3.
• Surface area of the oceans is exactly 3.61 × 108 km2.
• Initial mean temperature of the affected volume is 14°C.
• Density of sea water at various temperatures.
°C Density, g/mL
14 1.02512
15 1.02500
16 1.02483
17 1.02466
18 1.02449
• Assume that the temperature change is uniform throughout the entire volume under consideration.
• Assume that the surface area of the oceans does not change.