Biogeochemistry Problem Set
1. Natural sources add sulfur dioxide (SO2) to the atmosphere at a rate of about 108 metric tons of S per year. The background concentration of atmospheric SO2, measured in remote areas where anthropogenic sources are not likely to have much influence, is about 0.2 parts per billion, by volume [ppb(v)]. What is the residence time, in days, of atmospheric SO2 in remote regions? Useful info: molar mass of air = 28.96 g/mol, mass of Earth's atmosphere is 5.14 x 1021 g, and ppb(v) is equivalent to mole fraction.
2. A stable and highly soluble pollutant is dumped into a lake at the rate of 0.16 metric tons per day. The lake volume is 4 x 107 m3 and the average water flow-through rate is 8 x 104 m3 /day. Ignore evaporation from the lake surface and assume the pollutant is uniformly mixed in the lake. What eventual steady-state concentration (in ppm) will the pollutant reach?
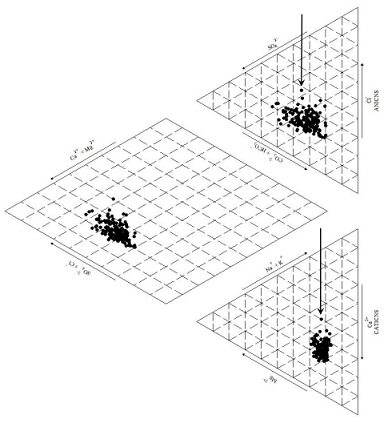
3. Examine the Piper Diagram on the following page characterizing the chemistry of a locally well-known waterway.
(a) Based on where the majority of points plot on the diagram, classify the type of water chemistry represented by these samples.
(b) For the INDICATED DATA POINT (WITH ARROW), what are the percentages of each ion?
|
Cation
|
Percentage
|
Anion
|
Percentage
|
|
Ca2+
|
|
SO42-
|
|
|
Mg2+
|
|
Cl-
|
|
|
Na+ + K+
|
|
CO32- + HCO3-
|
|
(c) On the diagram, PLOT a point on each part representing a water sample having the following percent ions: Ca2+ = 70, Mg2+ = 20, (Na+ + K+) = 10, SO42- = 60, Cl- = 5, and (CO32- + HCO3-) = 35.