BIOCHEMISTRY HOMEWORK
Question 1 - Carbon monoxide (CO) is a potentially fatal poison. Amongst other things, CO exposure causes anoxemia, a reduced oxygen concentration in blood. CO binds avidly to hemoglobin (Hb), to form carboxyhemoglobin, COHb. The diagram shows plots of the oxygen content of whole blood as a function of the partial pressure of oxygen. Curves are shown for normal blood, for blood containing 50% COHb, and for blood from a patient with 50% anemia (that is half the normal content of hemoglobin).
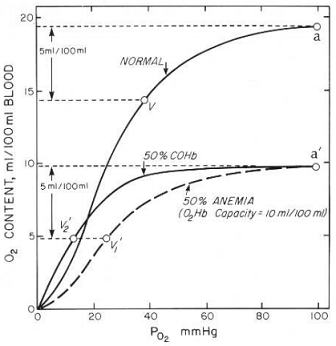
(a) Describe the two major differences between the oxygen binding curves for normal blood and for blood containing 50% COHb?
(b) What is the binding site for CO in hemoglobin?
(c) On the basis of the shape of the curve for 50% COHb, how would you best describe the mode of action of CO (based on what you have learned in class)? Bearing in mind your answer to question (b), is this description of the behavior of CO completely correct?
(d) Explain the two reasons why CO restricts the delivery of oxygen to muscles.
(e) Is CO poisoning (to a level of 50% COHb) more or less severe than 50% anemia, in terms of the ability of blood to deliver O2 to tissues?
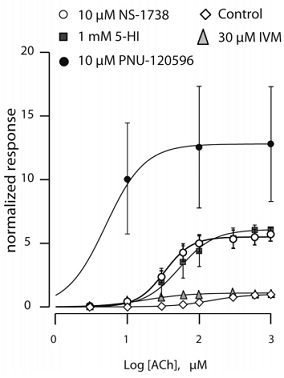
Question 2 - The α7 nicotinic acetylcholine receptor (nAChR) is a ligand-gated ion channel expressed in the mammalian central nervous system. In response to acetylcholine (Ach), the channel mediates increase in intracellular free calcium levels. Gene knock-out and antisense studies together with pharmacological studies using small-molecule modulators have demonstrated that α7 nAChR plays an important role in aspects of attention, cognition, and neuroprotection relevant to diseases such as schizophrenia and Alzheimer's disease. Positive modulators selective for the α7 nAChR show efficacy in animal models of cognitive dysfunction.
Effects of four compounds, ivermectin (IVM), 5- hydroxyindole (5-HI), 1-(5-chloro-2-hydroxyphenyl)-3- (2-chloro-5-trifluoromethylphenyl)urea (NS-1738) and 1-(5-chloro-2,4-dimethoxyphenyl)-3-(5- methylisoxazol-3-yl)urea (PNU-120596), which act as positive allosteric modulators selective for α7 nAChR, have been studied in Xenopus laevis oocytes. Receptor activity was correlated with an increase in intracellular free calcium levels in the presence of the modulator compounds and plotted as normalized response to acetyl choline (ACh). Explain the data shown in the graph below by ranking the effects of the compounds and comment on why there are differences in the profile of allosteric modulation between compounds. Your answer should include a description of allostery and how allosteric modulation is different than the action of agonist and antagonist compounds.
Question 3 - How is the rate of glycolysis controlled? Your answer should mention the reaction steps and corresponding enzymes that are subjected to regulation and the biochemical and thermodynamic basis of glycolysis control.
Question 4 - How does glucose enter in eukaryotic cells? Describe the biochemical specificity and driving-force of the processes.
Question 5 - Triose phosphate isomerase interconverts dihydroxyacetone phosphate and glyceraldehyde-3-phosphate. Mutants of yeast or bacteria that lack triose phosphate isomerase are viable under aerobic conditions but cannot grow in the absence of oxygen. Explain (a) why these mutants can grow aerobically and (b) why they cannot grow anaerobically.
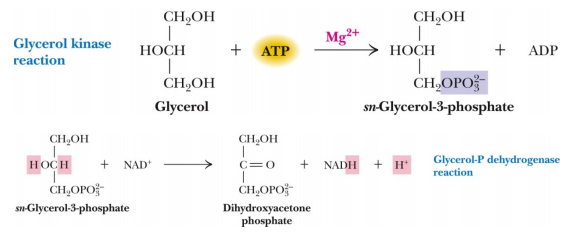
Question 6 - Glycerol is a sugar alcohol that can be used as a source of carbon and energy by many microorganisms growing aerobically. The diagram below shows the first two steps of glycerol catabolism, note that one ATP is consumed and one NADH is produced in these two reactions, per molecule of glycerol.
Glycerol cannot be used as an energy source by organisms growing anaerobically using either the lactate or the ethanol fermentation pathway. Why not?
Question 7 - Describe favism and associated pathophysiology.