Assignment
Part 1: Identifying Types of Reactions
1. Classification of chemical reactions(2016-2017)
1. Matching: Match the following statements to one of the five types of reactions it best describes. Place the letters from the right column in the blocks on the left column.
- Two compounds react to form two different compounds.
- Oxygen combines with a hydrocarbon to form water and carbon dioxide.
- A compound reacts with an element to form a new compound and a different element.
- One reactant is broken down into two or more products.
- Two or more reactants combine to form one product.
A. Combustion
B. Decomposition
C. Synthesis (Combination)
D. Single replacement
E. Double replacement
2. Review and Practice: Balance the 10 chemical equations below (labeled A-J). (Some equations may only need coefficients of 1 so they appear balanced without anything added. You may put in the 1's if it helps you.)In the space to the right, classify the reaction as a synthesis,decomposition, single replacement, double replacement, or combustion reaction.
Reaction(you must balance each - don't change subscripts) Type of Reaction
A. Mercury (I) Oxide forms mercury and oxygen.
_____ Hg2O → _____ Hg + _____ O2 _____
B. Cesium nitrate and potassium chloride react to produce cesium chloride and potassium nitrate.
_____ CsNO3 + _____ KCl → _____ CsCl + _____ KNO3 _____
C. Hexose (a type of sugar)burns in oxygen to produce water andcarbon dioxide.
_____ C6H12O6 + _____ O2 → _____ H2O + _____ CO2 _____
D. Magnesiumhydrogen carbonate is heated to producemagnesium carbonate and carbon dioxide andwater.
_____ Mg(HCO3)2 → _____ MgCO3 + _____ CO2 + _____ H2O _____
E. Pentane burns in oxygen to formcarbon dioxide and water.
_____ C5H12 + _____ O2 → _____ CO2 + _____ H2O _____
F. Calcium chloride and sodium sulfate produce calcium sulfate and sodium chloride.
_____ CaCl2 + _____ Na2SO4 → _____ CaSO4 + _____ NaCl _____
G. Water and selenium trioxide react to produce selenic acid.
_____ H2O + _____ SeO3 → _____ H2SeO4 _____
H. Lithium iodide and bromine react to produce lithium bromide and iodine.
_____ LiI + _____ Br2 → _____ LiBr + _____ I2 _____
I. Calcium and hydrochloric acid produce calcium chloride and hydrogen gas.
_____ Ca + _____ HCl → _____ CaCl2 + _____ H2
J. Aluminum and copper (II) chloride produces aluminum chloride and copper.
_____ Al + _____ CuCl2 → _____ AlCl3 + _____ Cu _____
Part 2: Stoichiometry Worksheet
Directions: You are strongly encouraged to print out this worksheet and work with paper&pencil and a calculator for these problems. Then, either scan in your completed worksheet or record your answers on this sheet and attach to it the drop box. You must show work for credit. There are 10 questions. SHOW THE SET-UP OF YOUR SOLUTION.
Example Problem 1. Use this balanced equation: 2 C2H6 + 7 O2 → 4 CO2 + 6 H2O.
Ex. How many grams of Water are produced when 10 grams of C2H6 is burned?
Set up the problem to go from the starting value to the ending value (what is given to what is needed).
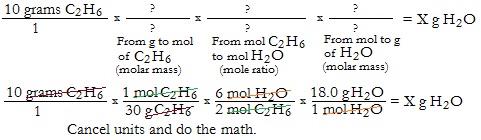
Notice that the answer is 18 grams
Did you calculate? Did you get the right answer?
Use the following equation to answer questions 1-3: 2 C4H10 + 13 O2 → 8 CO2 + 10 H2O
1. How many moles of H2O are produced when 2 moles of C4H10 are burned?
2. How many moles of Oxygen are needed to completely burn 2 moles C4H10?
3. How moles of CO2 are produced when 2 moles C4H10 are completely burned?
Use the following equation to answer questions 4 & 5: 6 HCl + 2 Al → 2 AlCl3 + 3 H2
4. How many moles of Aluminum are needed to produce 4 moles of AlCl3?
5. How many moles of HCl are needed to use up 1 mole of Al?
Use this equation to answer questions 6 & 7: 2 C4H10 + 13 O2 → 8 CO2 + 10 H2O
6. How many moles of O2 are needed when 16 moles of C4H10react?
7. How many moles of CO2 are produced when 6.0 moles of C4H10 react?
Use this equation to answer question 8. 2 K + Br2 → 2 KBr
8. How many moles of KBr are produced from 4.50 g of K?
Use the following balanced equation to answer 9 & 10. 2 H2 + O2 → 2 H2O
9. How many grams of H2O are produced when 8.0 grams of O2 react?
10. How many moles of hydrogen (H2) are needed to react with8.0 g of O2?
Part 3: Stoichiometry and Limiting Reagents
Answer the following 15 questions.
1. Stoichiometry problems use all of the following, except one. Which one is NOT involved in stoichiometry?
a. mass of reactants or products
b. number of moles of reactants or products
c. amount of time it takes for a reaction to occur
d. a balanced equation must be used to solve stoichiometry problems
2. When the lesson pages talk about a mole ratio between two substances, what does that mean? Where do the numbers for the mole ratio come from?
3. One mole of Aluminum may be represented as 1 mol or _____ grams or _____ atoms.
4. Short essay question. Why is a balanced chemical equation needed to solve a stoichiometry problem?
5. Consider the reaction of combining hydrogen and nitrogen to produce ammonia.
The balanced equation is: 3H2 + N2 → 2NH3
a. What is the mole ratio of hydrogen to nitrogen?
b. What is the mole ratio of hydrogen to ammonia?
c. What is the mole ratio of nitrogen to ammonia?
6. Consider the equation HCl + Al → AlCl3 + H2.
a. Is this equation balanced?
b. Balance it. _ HCl + _ Al → _ AlCl3 + _ H2
7. Use the balanced equation in #6b above. How many moles of hydrogen would be produced if you used 12 moles of HCl and enough Al to completely react?
8. In your own words, explain one of the methods used, (either from MrHunyady's video explanations, or the other method from the stoichiometry lesson) of how to find the amount of grams of a product from a given amount of moles of a reactant? This is an essay question. Please use an example to show how it works.
9. Explain what we mean by a limiting reagent. Use an everyday example to show what this means.
10. Suppose you are going to make sandwiches that contain 2 pieces of bread, 2 slices of ham, 3 slices of turkey, 2 leaves of lettuce and 2 pieces of cheese. If you have a total of 60 pieces of bread, 40 slices of ham, 30 slices of turkey, 50 leaves of lettuce and 20 pieces of cheese, how many sandwiches can you make?
11. In the sandwich problem above, how many pieces of ham would be left over after making all the sandwiches you could?
12. What is the limiting reagent (or ingredient) in this sandwich problem? (Is there more than one?)
13. Describe, in your own words, what is meant be each of these terms:
a. actual yield
b. theoretical yield
c. percent yield
14. Why would chemists not get a 100% yield on a reaction they run in a laboratory?
15. If a student calculates that he should get exactly 400.0 g of a certain product but in the lab, he only gets 285 g, what is the percent yield for the reaction?