Assignment: Introduction to Chemistry Survey
LAB: Mixtures & Capillary Action
Introduction
There is much more to the color of a pen or marker than it may seem. Almost all colors are the result of mixing pigments. A mixture is a combination of two or more substances that do not change one another's molecular structure. The molecules of the substances in the mixture stay the same. A simple example of a mixture is trial mix. Even though there are many things in the trail mix, like nuts, raisins, and seeds - each item stays the same and can be separated from the mixture.Sometimes mixtures appear to be a single substance, but scientists can perform experiments to separate the ingredients in a mixture and see what it is really made of. In this experiment, you are going to separate the color mixtures in ink.
Experimental Procedure
Materials needed
• Pipe cleaner
• 3 different pens or markers
• Paper coffee filter
• Cup of water
Procedure
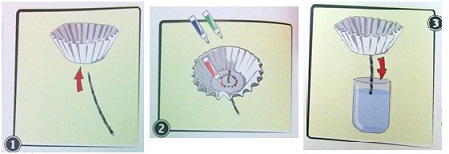
1. Poke a pipe cleaner through the center of a coffee filter.
2. Use three different pens or markers to make a circle of dots around the center of the filter.
3. Place the pipe cleaner end in a cup of water, with the coffee filter at the top. Observe the coffee filter for about 5 minutes. See Experimental Results & Observationssection to record your results. If no separation of colors occurred, repeat experiment using different color pens or markers!
References
Adapted from: Big Book of Science Experiments. Rosenbloom, J. Ed. 2011 Time Home Entertainment Inc. NY, NY.
Experiment: Capillary Action
1. Describe "capillary action."
2. Describe "chromatography."
3. Describe what you observed with Pen #1. Color/type of pen, i.e. red sharpie permanent marker. How many spots? How far had each spot moved from its original position? What color was the spot? (you may draw a sketch of your observations on the back to help with your description)
4. Describe what you observed with Pen #2 as you did with pen #1.
5. Describe what you observed with Pen #3 as you did with pen #1.
6. Which pen had ink that was a mixture of the most colors? What were the colors?
7. Which pen had ink colors that moved the farthest?
8. What causes the movement of ink on the filter?
9. How does this experiment relate to capillary action?