Assignment -1
In nucleophilic substitution bimolecular(SN2) reactions of alkyl halides, the order of reactivity is: R-I>R-Br>R-Cl>R-F, where R is an alkyl group. Alkyl Iodides are around 105 to 106 times more reactive than alkyl fluorides.
Contrary to this, 1-halo-2,4-dinitrobenzenes react at almost the same rate, in nucleophilic aromatic substitution. What could be the possible reason for this difference in the reactivity of haloalkanes and substituted haloarenes?
Assignment -2
What is the pH of a buffer system consisting of 0.30 MNH3 and 0.35 MNH4Cl buffer. What is the change in pH after the addition of 20.0 mL of 0.050 M NaOH to 70.0 mL of this buffer solution?
(Kb of NH3 = 1.8 x10-5)
Assignment -3
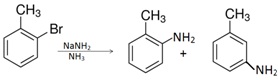
Aryl halides undergo nucleophilic substitution in presence of a strong base such as sodium amide in liq.ammonia. If o-bormotoluene is made to react with this base, o-toluidine and m-toludine are formed in equal amounts instesd of m-toluidine being the major prodict. How do you account for this fact?
Assignment -4
In an experiment, a 10 g piece of iron (specific heat = 0.45 J/g 0C) at 100 °C is dropped into 25 g of water (specific heat = 4.2 J/g 0C) at 27 degree C. Find temperature of the iron and water system at thermal equilibrium.
Assighment- 5
In an experiment, 25 mL of 0.10 M acetic acid is titrated with 0.10 sodium hydroxide solution. What is the pH of the solution after the addition of 25 mL of NaOH. (ionization constant of the conjugate base = 5.6x10-10)