1. List all different electromagnetic spectrum regions indicating the range of frequency, wave length and energy of each region. Identify the kind of molecular transitions that correspond to each electromagnetic region.
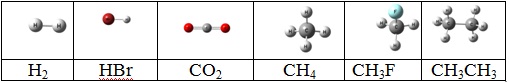
2. For each of the following molecules:
a) Redraw each molecule and find the point group for each one.
b) Draw all symmetry elements on each molecule.
c) Show which one will give rise to microwave or far infrared pure rotational spectrum indicating the selection rules for each molecule.
d) Show which one will give rise to infrared vibrational rotational spectra indicating the selection rules for each molecule?
3. Rotational absorption lines from 1H35Cl were found at the following wavenumbers: 83.32, 104.13, 124.73, 145.37, 165.89, 186.23, 206.60, 226.86 cm-1.
a) Use any statistical program (a spread sheet program would be enough) to analyze this spectrum to find B and DJ.
b) Calculate the bond length of the molecule.
c) What is the transition with the highest intensity at room temperature?
d) Predict the fundamental vibration frequency of 1H35Cl.
e) What would be the bond length of the molecule 2H35Cl?
4. For the molecule C2H4 which belongs to the D2d point group:
a) How many vibrational modes does the molecule have? How many of these modes would be stretching and how many would be bending modes?
b) What irreducible representations would the IR and the Raman active modes take?
c) Show on the molecule one of the IR active modes that is parallel and indicate the selection rules for the vibrational rotational transition of this mode.
d) Show on the molecule one of the IR active modes that is perpendicular and indicate the selection rules for the vibrational rotational transition of this mode.